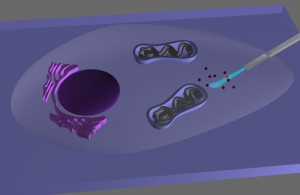
 An endoscope that can provide high-resolution optical images of the interior of a single living cell, or precisely deliver genes, proteins, therapeutic drugs or other cargo without injuring or damaging the cell, has been developed by researchers from Berkeley Lab and the University of California (UC) Berkeley.
An endoscope that can provide high-resolution optical images of the interior of a single living cell, or precisely deliver genes, proteins, therapeutic drugs or other cargo without injuring or damaging the cell, has been developed by researchers from Berkeley Lab and the University of California (UC) Berkeley.
The researchers attached a tin-oxide nanowire waveguide to the tapered end of an optical fiber to create a novel endoscope system. Light traveling along the optical fiber can be effectively coupled into the nanowire, where it is re-emitted into free space when it reaches the tip.
The nanowire tip is extremely flexible due to its small size and high aspect ratio, yet can endure repeated bending and buckling so that it can be used multiple times.
“By combining the advantages of nanowire waveguides and fiber-optic fluorescence imaging, we can manipulate light at the nanoscale inside living cells for studying biological processes within single living cells with high spatial and temporal resolution,”
Peidong Yang, a chemist with Berkeley Lab’s Materials Sciences Division, who led this research.
Despite significant advancements in electron and scanning probe microscopy, visible light microscopy remains the workhorse for the study of biological cells. Because cells are optically transparent, they can be noninvasively imaged with visible light in three-dimensions. Also, visible light allows the fluorescent tagging and detection of cellular constituents, such as proteins, nucleic acids and lipids.
The one drawback to visible light imaging in biology has been the diffraction barrier, which prevents visible light from resolving structures smaller than half the wavelength of the incident light. Recent breakthroughs in nanophotonics have made it possible to overcome this barrier and bring subcellular components into view with optical imaging systems. However, such systems are complex, expensive, and bulky in size.
“Previously, we had shown that subwavelength dielectric nanowire waveguides can efficiently shuttle ultraviolet and visible light in air and fluidic media. By incorporating one of our nanophotonic components into a simple, low-cost, bench-top fiber-optical set-up, we were able to miniaturize our endoscopic system.”
Peidong Yang
To test their nanowire endoscope as a local light source for subcellular imaging, Yang and his co-authors optically coupled it to an excitation laser, then waveguided blue light across the membrane and into the interiors of individual HeLa cells, the most commonly used immortalized human cell line for scientific research.
“The optical output from the endoscope emission was closely confined to the nanowire tip and thereby offered highly directional and localized illumination. The insertion of our tin oxide nanowire into the cell cytoplasm did not induce cell death, apoptosis, significant cellular stress, or membrane rupture. Moreover, illuminating the intracellular environment of HeLa cells with blue light using the nanoprobe did not harm the cells because the illumination volume was so small, down to the picoliter-scale.”
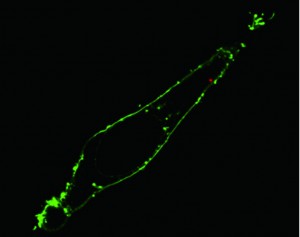 Delivering payloads into the cell
Delivering payloads into the cell
The researchers also tested the endoscope’s capabilities for delivering payloads to specific sites inside a cell. While carbon and boron nitride nanotube-based single-cell delivery systems have been reported, these systems suffer from delivery times that range from 20 to 30 minutes, plus a lack of temporal control over the delivery process.
To overcome these limitations, they attached quantum dots to the tin oxide nanowire tip of their endoscope using photo-activated linkers that can be cleaved by low-power ultraviolet radiation. Within one minute, their functionalized nanowire endoscope was able to release its quantum dot cargo into the targeted intracellular sites.
“Confocal microscopy scanning of the cell confirmed that the quantum dots were successfully delivered past the fluorescently labeled membrane and into the cytoplasm. Photoactivation to release the dots had no significant effect on cell viability.”
The highly directional blue laser light was used to excite one of two quantum dot clusters that were located only two micrometers apart. With the tight illumination area and small separation between the light source and the dots, low background fluorescence and high imaging contrast were ensured.
“In the future, in addition to optical imaging and cargo delivery, we could also use this nanowire endoscope to electrically or optically stimulate a living cell”. – Yang
Links
Ruoxue Yan, et al., Nanowire-based single-cell endoscopy, Nature Nanotechnology, 2011
Abstract for the above paper:
One-dimensional smart probes based on nanowires and nanotubes that can safely penetrate the plasma membrane and enter biological cells are potentially useful in high-resolutionand high-throughputgene and drug delivery, biosensingand single-cell electrophysiology. However, using such probes for optical communication across the cellular membrane at the subwavelength level remains limited. Here, we show that a nanowire waveguide attached to the tapered tip of an optical fibre can guide visible light into intracellular compartments of a living mammalian cell, and can also detect optical signals from subcellular regions with high spatial resolution. Furthermore, we show that through light-activated mechanisms the endoscope can deliver payloads into cells with spatial and temporal specificity. Moreover, insertion of the endoscope into cells and illumination of the guided laser did not induce any significant toxicity in the cells.